A Sham Controlled Randomized Trial
The National Institute of Mental Health funded an independent, randomized, controlled trial in which 190 patients were treated utilizing NeuroStar Advanced Therapy TMS for 4 – 6 weeks. The trial found that patients who received NeuroStar Advanced Therapy TMS were four times more likely to achieve remission than the patients who received a sham treatment. 50% of patients experienced significant improvements in their depression symptoms and 33% experienced remission. Patients treated with NeuroStar Advanced Therapy also experienced significant improvement in anxiety and physical symptoms (such as appetite changes, aches and pains, and lack of energy) associated with depression.1
The Carpenter Trial
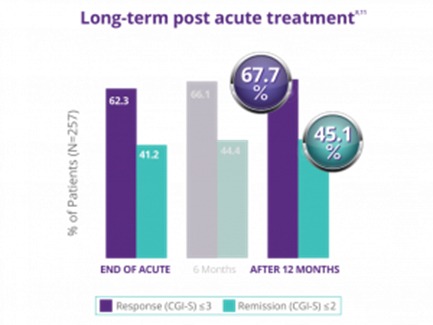
NeuroStar has demonstrated the durability of its effects established over 12 months..
In a trial with physician directed standard of care, meaning NeuroStar Advanced Therapy could be used in conjunction with antidepressants as needed, patients who had received treatment then reported their symptom levels at 3, 6, 9 and 12 months to determine the durability of their treatments. By the end of the 12-month period, 66% of patients who had either responded or completely remitted after TMS treatment remained at the symptom levels they reported at the end of the treatment phase.2
After the end of the treatment period, only 33% of patients needed to come back for maintenance TMS sessions, or ‘reintroduction’ during this 12-month period.[2]
___________________________________________
- George MS, et al. (2010). Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder: A Sham-Controlled Randomized Trial. Arch Gen Psychiatry, 67(5):507-516.
- Carpenter LL, et al. (2012). Depress Anxiety, 29(7):587-596.
The NeuroStar Advanced Therapy System is indicated for the treatment of depressive episodes and for decreasing anxiety symptoms for those who may exhibit comorbid anxiety symptoms in adult patients suffering from Major Depressive Disorder (MDD) and who failed to achieve satisfactory improvement from previous antidepressant medication treatment in the current episode.
The NeuroStar Advanced Therapy system is intended to be used as an adjunct for the treatment of adult patients suffering from Obsessive-Compulsive Disorder (OCD).
NeuroStar Advanced Therapy is only available by prescription. A doctor can help decide if NeuroStar Advanced Therapy is right for you. Patients’ results may vary.
The most common side effect is pain or discomfort at or near the treatment site. These events are transient; they occur during the TMS treatment course and do not occur for most patients after the first week of treatment. There is a rare risk of seizure associated with the use of TMS therapy (<0.1% per patient).
Visit neurostar.com for full safety and prescribing information.

