Safety
The most common side effect reported during other clinical trials was scalp discomfort – generally mild to moderate and occurring less frequently after the first week of treatment. Less than 5% of patients discontinued treatment NeuroStar Advanced Therapy TMS due to adverse events.
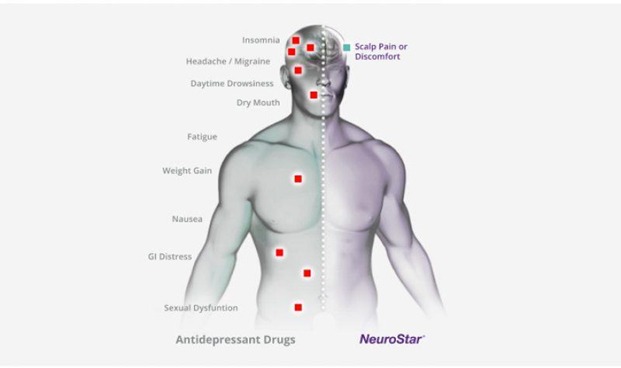
When it comes to side effects:
- NeuroStar Advanced Therapy TMS should not be used in patients with implanted metallic devices or non-removable metallic objects in or around the head. This does not include metallic fillings in teeth.
- NeuroStar Advanced Therapy TMS should not be used in patients with implants controlled by physiological signals. This includes pacemakers, implantable cardioverter defibrillators (ICDs), and vagus nerve stimulators (VNS).
__________________________________________________
[1] Janicak, P, et al. Transcranial Magnetic Stimulation (TMS) in the Treatment of Major Depression: A Comprehensive Summary of Safety Experience from Acute Exposure, Extended Exposure and During Reintroduction Treatment. Journal of Clinical Psychiatry, February 2008.
The NeuroStar Advanced Therapy System is indicated for the treatment of depressive episodes and for decreasing anxiety symptoms for those who may exhibit comorbid anxiety symptoms in adult patients suffering from Major Depressive Disorder (MDD) and who failed to achieve satisfactory improvement from previous antidepressant medication treatment in the current episode.
The NeuroStar Advanced Therapy system is intended to be used as an adjunct for the treatment of adult patients suffering from Obsessive-Compulsive Disorder (OCD).
NeuroStar Advanced Therapy is only available by prescription. A doctor can help decide if NeuroStar Advanced Therapy is right for you. Patients’ results may vary.
The most common side effect is pain or discomfort at or near the treatment site. These events are transient; they occur during the TMS treatment course and do not occur for most patients after the first week of treatment. There is a rare risk of seizure associated with the use of TMS therapy (<0.1% per patient).
Visit neurostar.com for full safety and prescribing information.

