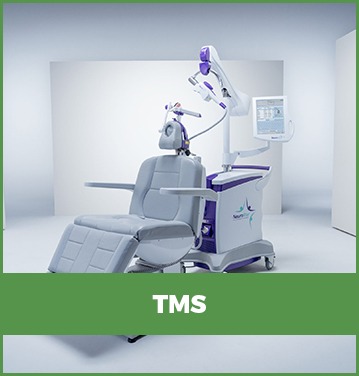
Transcranial Magnetic Stimulation (TMS), is a non-invasive
treatment for depression.

ABOUT US
Welcome to SNBCare!
SNBCare is a multidisciplinary group of dedicated professionals who provide the highest quality care with a wide range of outpatient mental health services. We have been serving adults, families, and couples for over a decade in a caring, patient-friendly environment. In providing behavioral health and mental health care services, we strive to integrate research evidence, clinical expertise and client values into our clinical practice. We recognize the individuality of all our patients and tailor services to meet their unique needs. Our experienced trusted staff members include licensed psychiatrists, psychiatric nurse practitioners and therapists. Together, this team of professionals uses the latest clinically proven methods and customized treatment plans for you or your loved one. With evidence-based treatment techniques that include individual, family, or couples therapy, cognitive behavioral therapy, insight-focused counseling, as well as psychiatric medicine, we take a holistic approach to your treatment.
ABOUT US
Welcome to SNBCare!

SNBCare is a multidisciplinary group of dedicated professionals who provide the highest quality care with a wide range of outpatient mental health services. We have been serving adults, families, and couples for over a decade in a caring, patient-friendly environment. In providing behavioral health and mental health care services, we strive to integrate research evidence, clinical expertise and client values into our clinical practice. We recognize the individuality of all our patients and tailor services to meet their unique needs. Our experienced trusted staff members include licensed psychiatrists, psychiatric nurse practitioners and therapists. Together, this team of professionals uses the latest clinically proven methods and customized treatment plans for you or your loved one. With evidence-based treatment techniques that include individual, family, or couples therapy, cognitive behavioral therapy, insight-focused counseling, as well as psychiatric medicine, we take a holistic approach to your treatment.
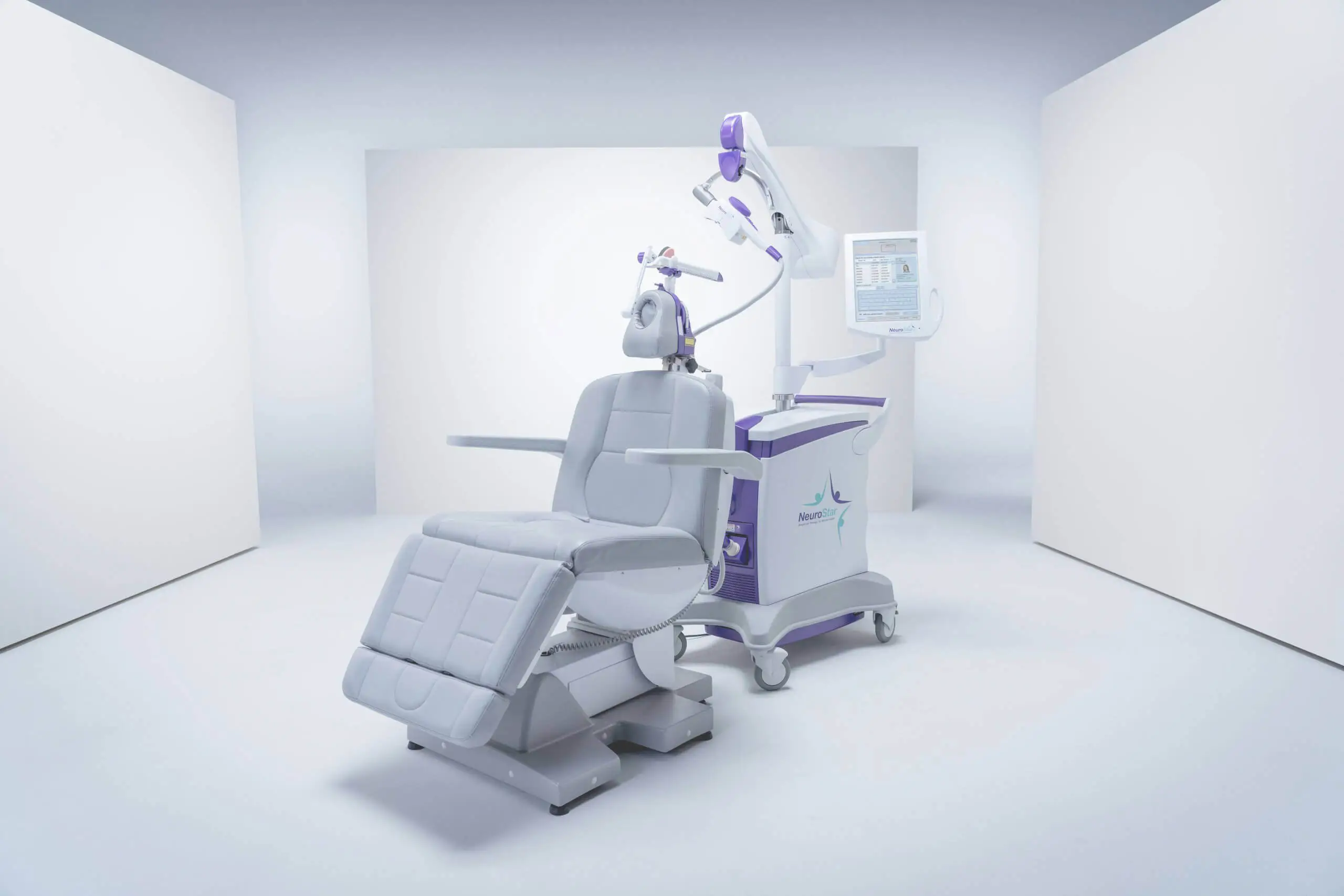




THERAPY
A breakthrough approach for those struggling with
treatment-resistant depression
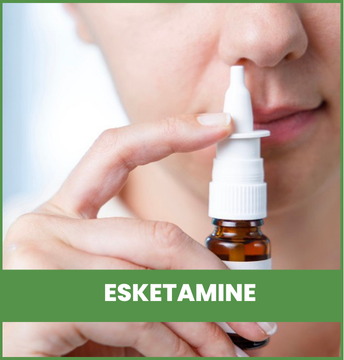
The careful selection of certain medications can enhance and
facilitate efficient symptom resolution
The use of digital information and communication tech,
to access health care services remotely





Transcranial Magnetic Stimulation
(TMS), is a non-invasive
treatment for depression.
THERAPY
A breakthrough approach for
those struggling with treatment-
resistant depression
The careful selection of
certain medications can
enhance and facilitate
efficient symptom resolution
The use of digital
information and communication
tech, to access health care
services remotely
Start your journey to mental wellness
We are here for you to get you back on track. Taking a step today will help lead to a better tomorrow. Call us today to make your appointment.
All information provided via our referral form is secure and confidential.
Accepted Insurances
SNBCare accepts most major insurances. For more information please contact our office.